What is H₂S?
Hydrogen sulfide (H₂S) is a colorless gas known for its characteristic rotten-egg smell. Its unpleasant odor is not the only problem it poses; H₂S is in fact highly toxic and corrosive, making it a serious problem in sectors such as wastewater treatment, biogas production, mining, aquaculture and oil refining.
How is H₂S formed?
H₂S is mainly generated by the decomposition of organic matter by bacteria in anaerobic (oxygen-deprived environments). This process, known as anaerobic digestion, is carried out by sulfate-reducing bacteria. These conditions occur naturally in wetlands, marine sediments and geothermal zones, but also in man-made systems such as sewers and methanizers. Also, in wastewater treatment systems and biogas plants, H₂S is a common by-product. Its formation is influenced by several factors:
- Type and quantity of organic matter
- Temperature and pH
- Flow dynamics and retention time
- Presence of sulfates and anaerobic bacteria
Because of its density (heavier than air), H₂S tends to accumulate in low spaces, presenting a high risk of sudden, concentrated exposure.
Problems caused by H₂S
Smells and nuisances
Even at very low concentrations, H₂S is detectable by smell. Complaints about H₂S odors are common in areas close to anaerobic digestion and wastewater treatment systems.
Toxicity
H₂S is a potent inhibitor of cellular respiration. Inhalation can affect the nervous system and lungs, causing symptoms ranging from headaches and nausea to loss of consciousness or death in the most severe cases. Even chronic exposure at low levels can cause health problems.
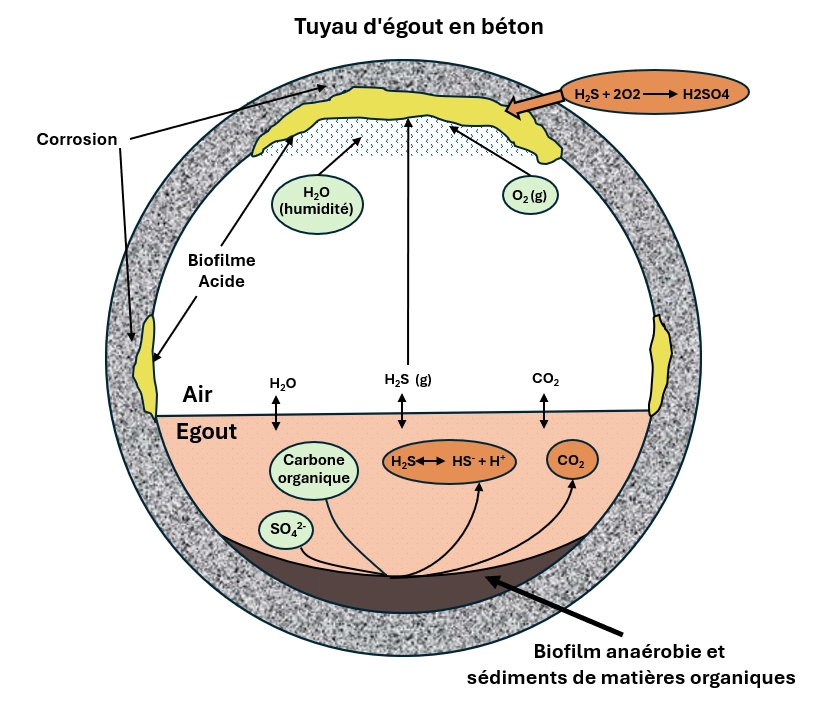
Corrosion and infrastructure damage
One of the most costly impacts of H₂S is corrosion. When H₂S encounters moisture, it can be oxidized by bacteria such as Thiobacillus thiooxidans into sulfuric acid (H₂SO₄). This acid aggressively attacks concrete and metal, resulting in:
- Accelerated degradation of pipes and equipment
- Frequent maintenance and repair
- Operational disruptions and increased costs
Concrete exposed to 20 ppm H₂S can deteriorate by 2-3cm every 5 years. Preventing the formation of H₂S is far more effective and sustainable than treating the symptoms.
How oxygen prevents the formation of H₂S
The principle
Oxygen plays an essential role in inhibiting the formation of H₂S. By maintaining aerobic conditions, we suppress the activity of sulfate-reducing bacteria, which are only active in anaerobic environments. In other words: no anaerobiosis, no H₂S
Why use oxygen?
Conventional treatments such as calcium nitrate, iron salts or sodium hypochlorite are commonly used to manage sulfides, but they have drawbacks: risks associated with chemical storage, recurring costs, sludge production and limited long-term effectiveness.
Pure oxygen, on the other hand, offers a clean, efficient and permanent solution. By directly increasing dissolved oxygen levels in the system:
- Anaerobic zones are eliminated.
- Sulfate-reducing bacteria are out-competed by aerobic microbes.
- H₂S formation is prevented at source, not just masked or neutralized after the fact.
Advantages of oxygen over traditional chemical treatments
In the biogas and wastewater treatment industries, oxygen dosing is increasingly recognized for its simplicity, reliability and effectiveness compared to chemical alternatives:
Treatment method |
Advantage |
Disadvantages |
| Calcium nitrates | Temporarily suppresses H₂S | High recurring cost, handling of chemicals |
| Iron chloride (FeCl3) | Reacts with H₂S to form solids | Sludge formation, interference with pH |
| Sodium hypochlorite | Oxide l₂S | Toxic by-products, safety hazards |
| Pure oxygen | Biologically prevents H₂S at source | Requires dosing system |
How to inject oxygen to prevent H₂S
Case 1: Biogas Production
In anaerobic digesters where biogas is generated from organic waste, H₂S is a common contaminant. To transform biogas into biomethane, H₂S must be removed.
Solution: Inject pure oxygen directly into the digester in microdoses. Oxygen promotes the growth of sulfur-oxidizing bacteria that convert H₂S to elemental sulfur, which can be separated.

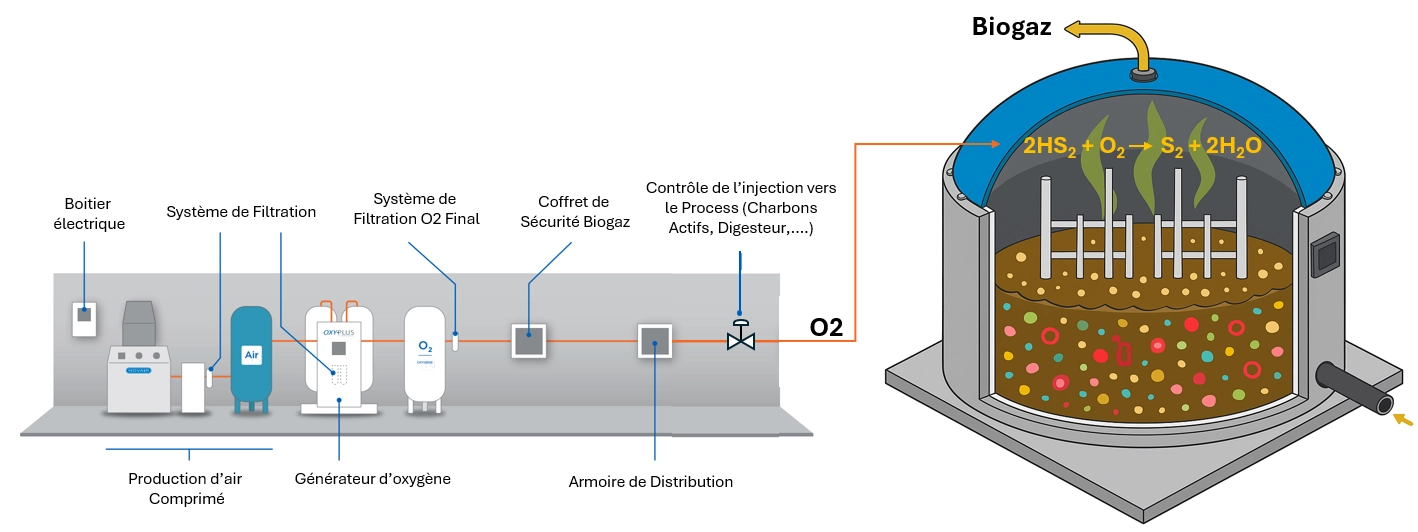
Main considerations:
- Oxygen dosage is precisely controlled (typically between 0.3% and 1% of biogas volume).
- A backflow prevention system ensures that biogas does not enter the oxygen storage.
- The process is continuous and integrates perfectly with gas upgrading technologies such as PSA, membranes or CO₂ recovery units.
Case 2: Wastewater and sewage networks
In backflow pipes and sewers, H₂S forms due to long retention times and stagnant, anaerobic conditions.
Solution: Use a superoxygenation system such as an oxygen cone to dissolve large amounts of oxygen in wastewater.
How it works:
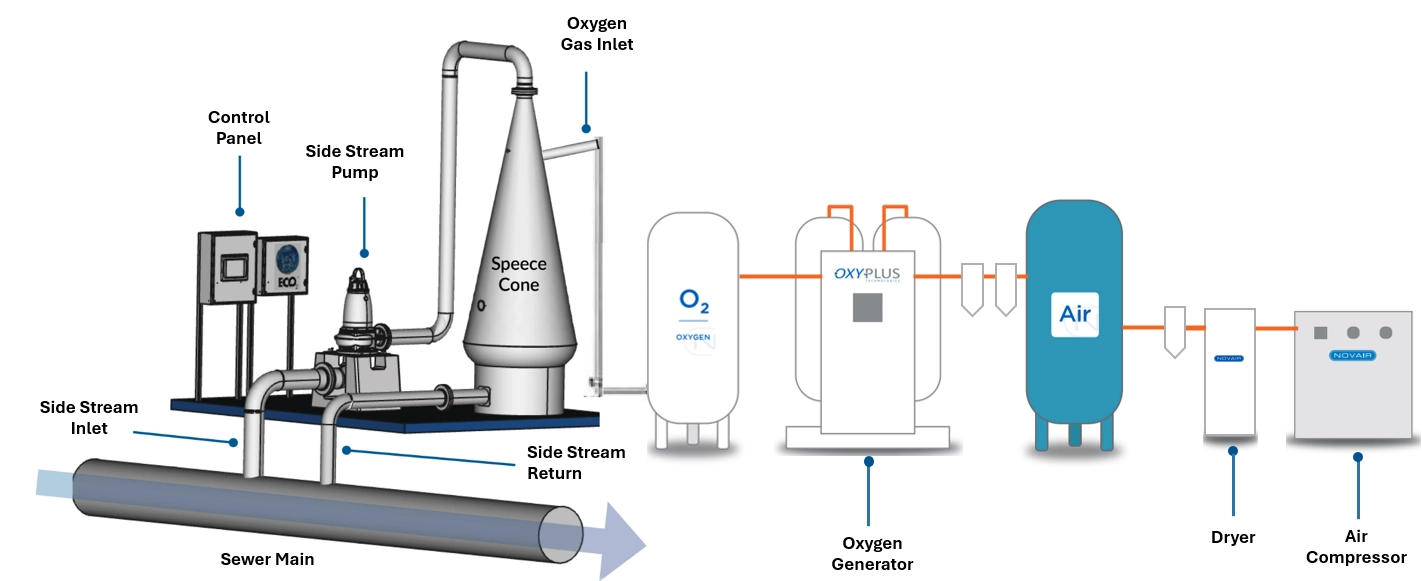
Example of an ECO2 cone system supplied with O2 by a NOVAIR generator
- A lateral flow of raw wastewater is pumped into a conically shaped dissolution chamber.
- Pure oxygen is injected, forming a dense cloud of microbubbles with a very large gas-liquid contact surface.
- The oxygen-rich flow is re-injected into the main sewer or force main, maintaining dissolved oxygen levels that prevent the formation of H₂S.
Benefits:
- Achieves high levels of OD
- Compact, energy-efficient system
- No need for chemical additives or frequent maintenance
Conclusion: choosing oxygen for clean, safe and sustainable H₂S control
Hydrogen sulfide is more than just an olfactory nuisance; it's a dangerous gas that threatens worker safety, infrastructure integrity and environmental quality. Preventing its formation is far more effective than trying to manage its consequences. Using pure oxygen to eliminate anaerobic conditions is a proven, clean and effective solution for biogas plants and sewage systems. It eliminates the root cause of H₂S, avoids the drawbacks of chemical treatments and promotes long-term sustainability.
Whether you operate a wastewater treatment facility, biogas plant or municipal sewer system, consider switching to oxygen-based prevention as your primary H₂S control strategy.

