Dissolved oxygen (DO) is a key element in many water-related systems: wastewater treatment plants, biological processes, aquaculture, etc.... The maximum concentration of oxygen that water can contain, known as the saturation level, depends on a number of physico-chemical factors. Understanding the influence of these parameters is essential for designing, optimizing or controlling systems where oxygenation plays a crucial role.
Temperature
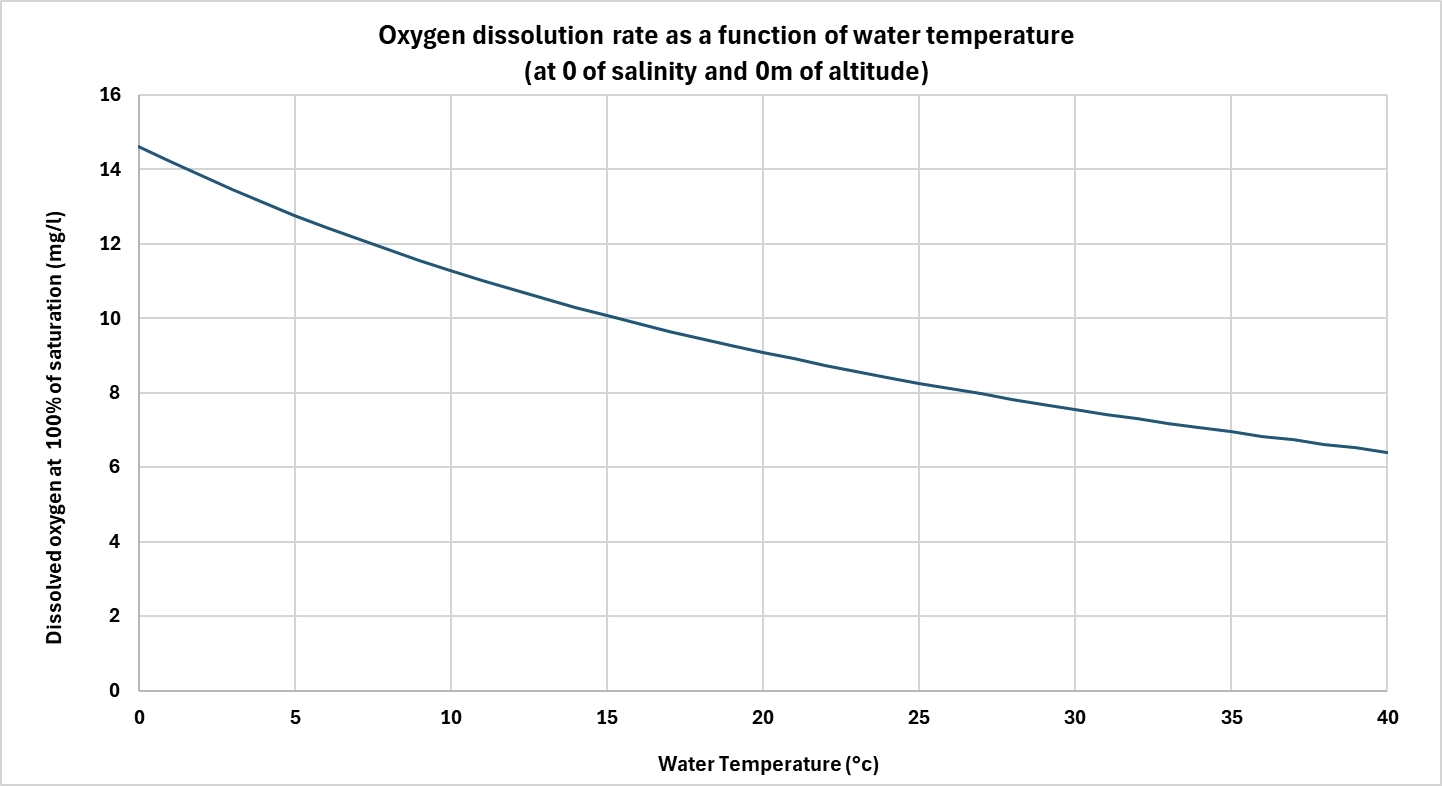
Water temperature is one of the most important parameters influencing oxygen solubility. The warmer the water, the less oxygen it can dissolve.
This phenomenon is explained by the thermal agitation of molecules: as temperature rises, it reduces interactions between water molecules and oxygen molecules, facilitating their release into the atmosphere. For example, at normal atmospheric pressure, fresh water can contain around 14.6 mg/L of oxygen at 0°C, compared with just 7.6 mg/L at 30°C.
In biological processes, this decrease in solubility can pose a problem, as oxygen requirements often increase with temperature (e.g. bacterial activity in wastewater treatment plants or RAS), while oxygen availability decreases. It then becomes necessary to compensate for this drop by improving diffusion or by supplying pure oxygen.
Salinity
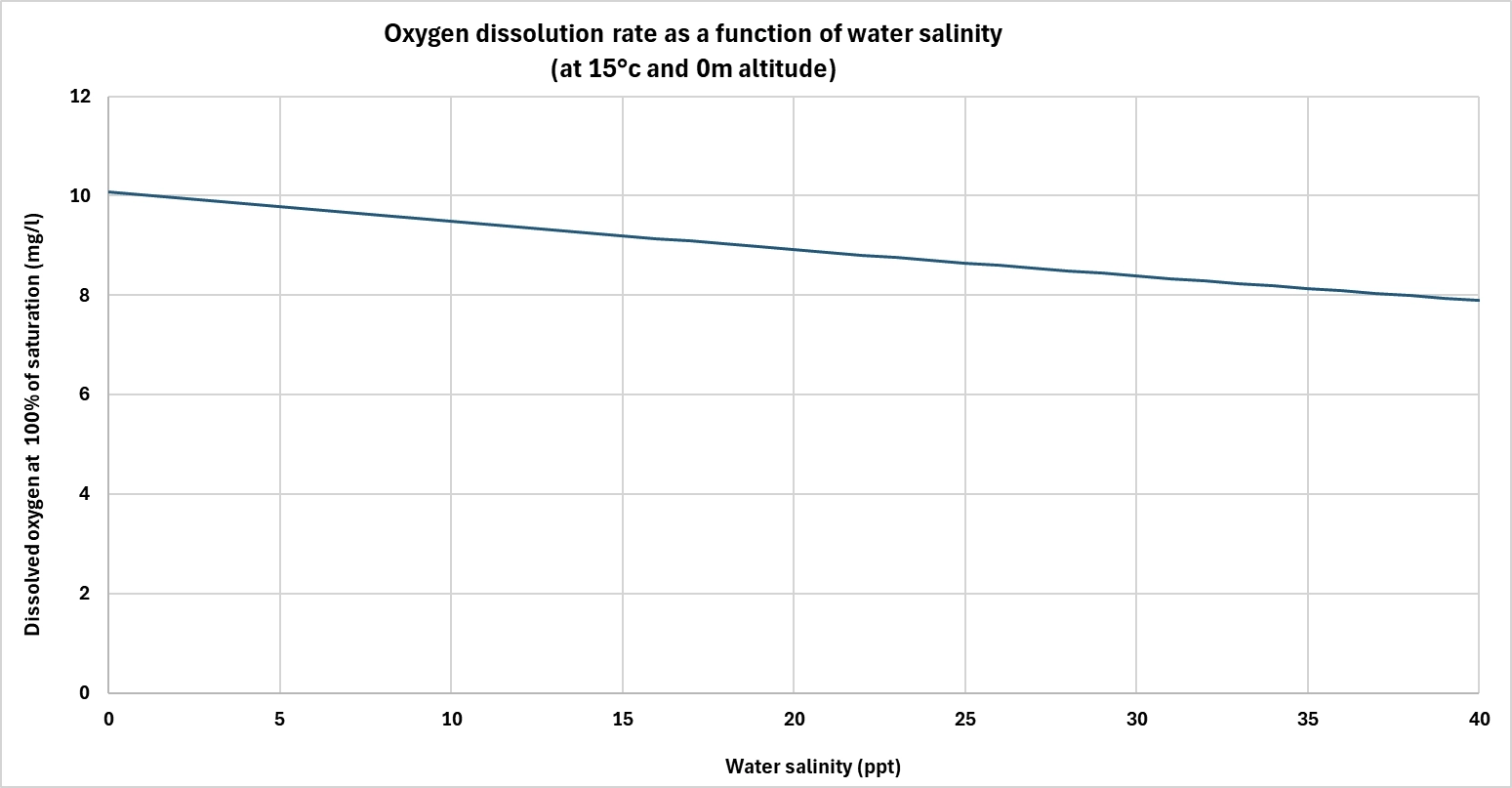
Salinity also plays an important role. The saltier the water, the less dissolved oxygen it can hold.
Dissolved salts take the place of gas molecules, reducing water's overall capacity to store oxygen. So, at constant temperature and pressure, seawater naturally contains less dissolved oxygen than freshwater.
This factor is particularly important when treating brackish or salty water, in industry or in coastal areas, where oxygenation strategies must be adapted to the specific physico-chemical conditions.
Pressure and altitude
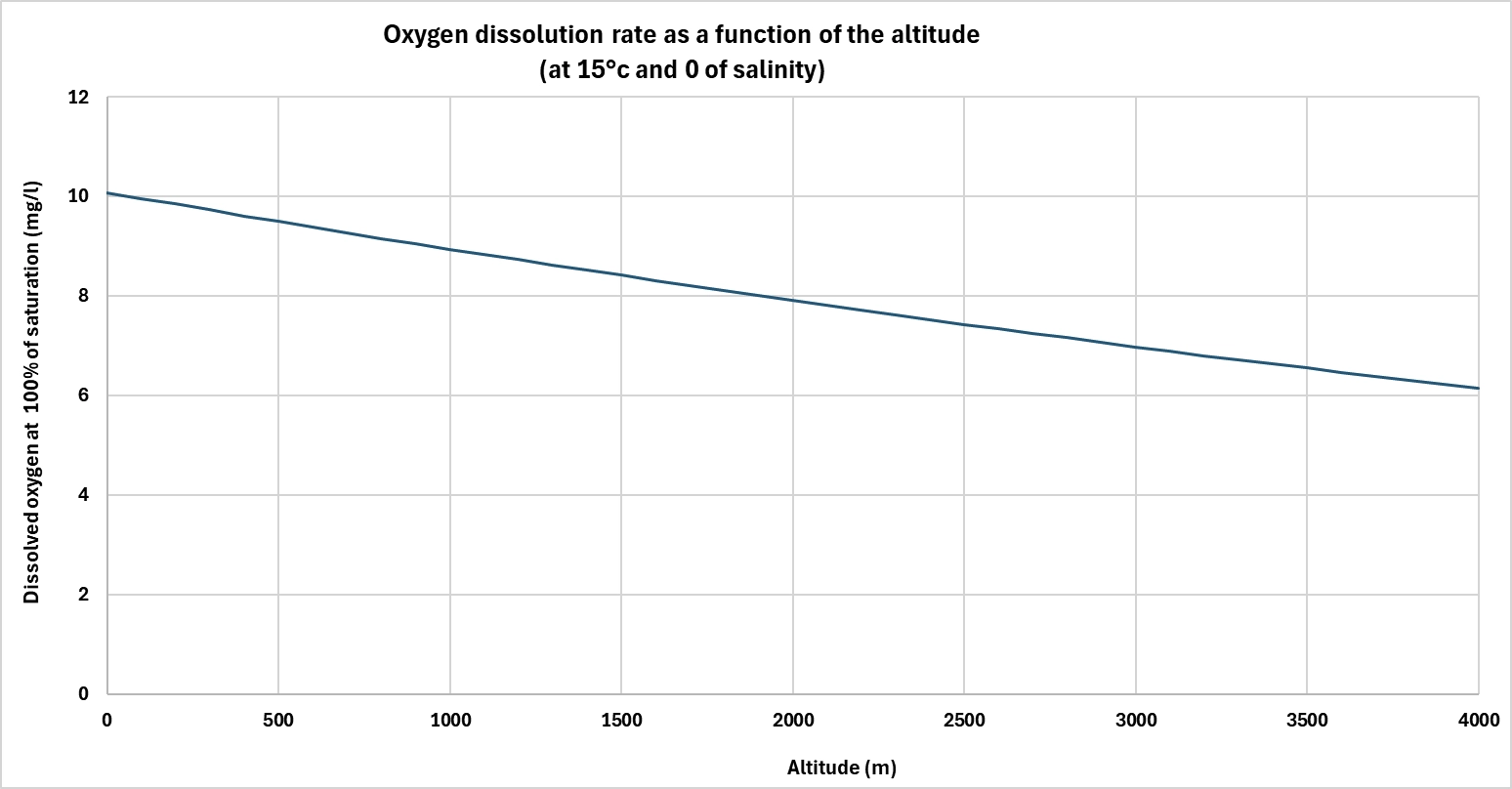
Atmospheric pressure directly affects the solubility of gases in water, including oxygen. The higher the pressure, the more oxygen water can dissolve.
At altitude, atmospheric pressure drops, reducing oxygen saturation capacity. Conversely, in a pressurized environment, solubility increases. This principle is exploited in a number of water treatment technologies, notably to obtain water supersaturated with oxygen.
For example, oxygen cones generally operate at a pressure of several bars. This overpressure enables much higher concentration levels to be achieved than would be possible at atmospheric pressure. Once the pressure is released, the water remains temporarily in a state of supersaturation, which can improve the effectiveness of treatments (oxidation in aquaculture, prevention of H₂S, biological filter,....).
Conclusion
Dissolved oxygen saturation is determined by well-known physical factors: temperature, salinity and pressure. Their influence is crucial in all water-related fields, such as aquaculture and wastewater treatment.
Whether it's to guarantee the proper operation of a wastewater treatment plant, maximize the yield of an oxidation process, limit odor nuisance in wastewater networks, or preserve an ecological balance, control of these parameters is an essential lever for efficient and appropriate oxygenation.

